When every single atom matters
Oxygen isotopes are extremely useful for tracing molecular processes, but the isotopes are rare and expensive. Prof. Christophe Copéret from the Laboratory of Inorganic Chemistry explains how his group makes chemistry with oxygen isotopes affordable. The key lies in the efficient synthesis of a small but energetic molecule: hydrogen peroxide.
Why are scientists interested in oxygen isotopes?
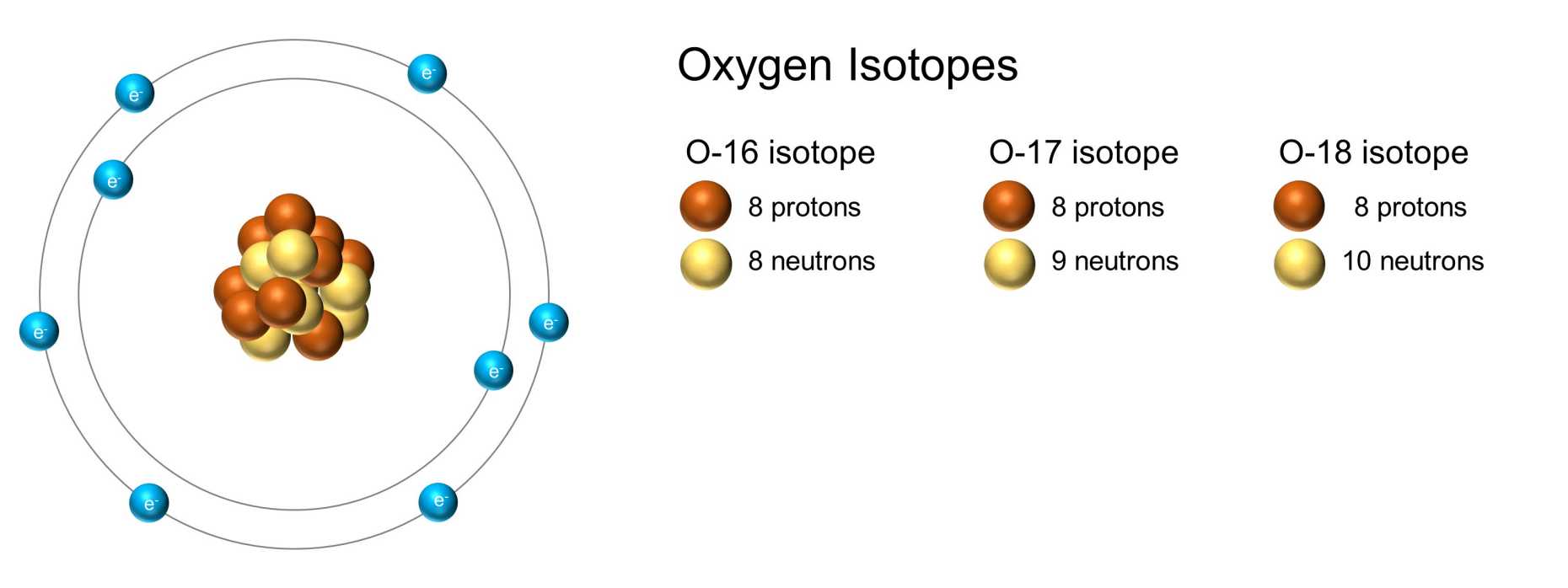
Oxygen isotopes, primarily O-17 and O-18, are used as tracers. Some examples: By replacing a specific oxygen atom with its isotope, a pharmacist can monitor the activity of drugs in cells. A synthetic chemist may seek a better understanding of a chemical process for optimising a production line. My own group uses isotopes for the study of catalytic reactions.

How do you replace an oxygen atom with its isotope?
There are various options. A very successful route is oxygen transfer by peroxides. Peroxides are very reactive compounds. We know peroxides from bleaching textiles, whitening teeth or sterilising surfaces. An isotope labeled peroxide will transfer its oxygen isotope very efficiently into a target molecule. We invented an elegant way to produce these isotopically labeled peroxides.
There are established industrial processes for the production of peroxides. What is special about your synthetic route?
Isotopes are a scarce and expensive resource. When producing peroxides from oxygen isotopes we do not waste a single isotope atom. The synthetic route is optimised in respect to the consumption of the isotope.
What makes your synthetic route so efficient? Is there a secret catalyst involved?
That is the funny part. Although my research group specialises in catalysis, there is absolutely no catalyst involved in this one. It is one of the quirks in science, which brought about this invention. When we prepare a catalyst for an experiment, we clean it first. We scrub off all unwanted oxygen from its surface using an organosilicon reagent. My collaborator, Keishi Yamamoto, saw the potential of our “cleaning” agent to produce peroxides very efficiently due to its affinity to oxygen. From this point on, we have used the organosilicon reagent as a precursor for the production of our own isotope labeled compounds. We filed a patent application on the synthetic route and we hope that the process will establish itself in academic as well as industrial research laboratories.
How big do you estimate the demand for your novel peroxide synthesis?
Peroxides are often unstable or even explosive. Scientists prepare them in situ, i.e. when, where and in the quantity that is needed. We believe that some laboratories have refrained from using O-tracing, because the overall process from obtaining the oxygen isotope and synthesising the peroxide is too elaborate and expensive. Our process is both easy in handling and cost-efficient. This makes O-tracing accessible to everyone.
What would a commercial product of your invention look like?
I envision it as a kit: isotopically labeled oxygen in a gas cylinder, organosilicon compound in a small vial. The customer mixes both together at room temperature under ambient pressure, stirs overnight, ready. We are looking for an industrial partner to take the invention to the market.

Professor Christophe Copéret
ETH Zurich, D-CHAB, Copéret Group
Contact / Links:
Professor Christophe Copéret, Copéret Group
Publication
“external page Activation of O2 by organosilicon reagents yields quantitative amounts of H2O2 or (Me3Si)2O2 for efficient O-transfer reactions”. K. Yamamoto et al., Helv. Chim. Acta 2018, 101,e1800156.
Patent
external page WO2019206744 (A1), EP 3 560 935 A1
Do you want to get more "News for Industry" stories?
external page Subscribe to our newsletter
external page Follow us on LinkedIn
Are you looking for research partners at ETH Zurich?
Contact ETH Industry Relations