Early diagnosis of resistant cancer
Treatment resistance accounts for 90% of cancer deaths and was hard to diagnose up to now. ETH spin-off Parithera enables early diagnosis.

We talked to CEO Antoine Herzog and CTO Weida Chen, the founders of Parithera.
Parithera advances circulating tumour cells (CTCs) analysis for cancer diagnostics. Why CTCs?
Antoine: By examining circulating tumour cells (CTCs), we get comprehensive information about the cancer. And all this in a minimally invasive way, which is very important for the patients.
All methods of diagnosing tumours available so far have significant disadvantages. Invasive methods like tissue biopsies – taking a sample from the tumour – lead to complications in 20% of patients. Our method belongs to the field of liquid biopsy – the process of sampling and analysing blood. Today, there are clinical methods to retrieve circulating tumour DNA via liquid biopsy. Tumour DNA is easier to detect, but it provides only limited information - much less than what we get by extracting and analysing intact circulating tumour cells at a single cell resolution.
Today there is no diagnostic performed with a single cell resolution. Why single-cell analysis?
Antoine: Cancer is very complex, so the diagnostics need to be very complex, too. Our enterprise is about earlier and easier diagnosis of resistance against treatment and automating this diagnostic process.
Imagine the tumour as a bowl of strawberries. One is rotten at the bottom of the bowl, that’s a resistance cell. Today’s diagnostic is based on a smoothie from blended strawberries. There is no way to tell if one cell was rotten. We are developing a set of technologies to keep those strawberries intact and look at them one by one. That way we can determine exactly what’s going on in these cells.
What are the difficulties you face?
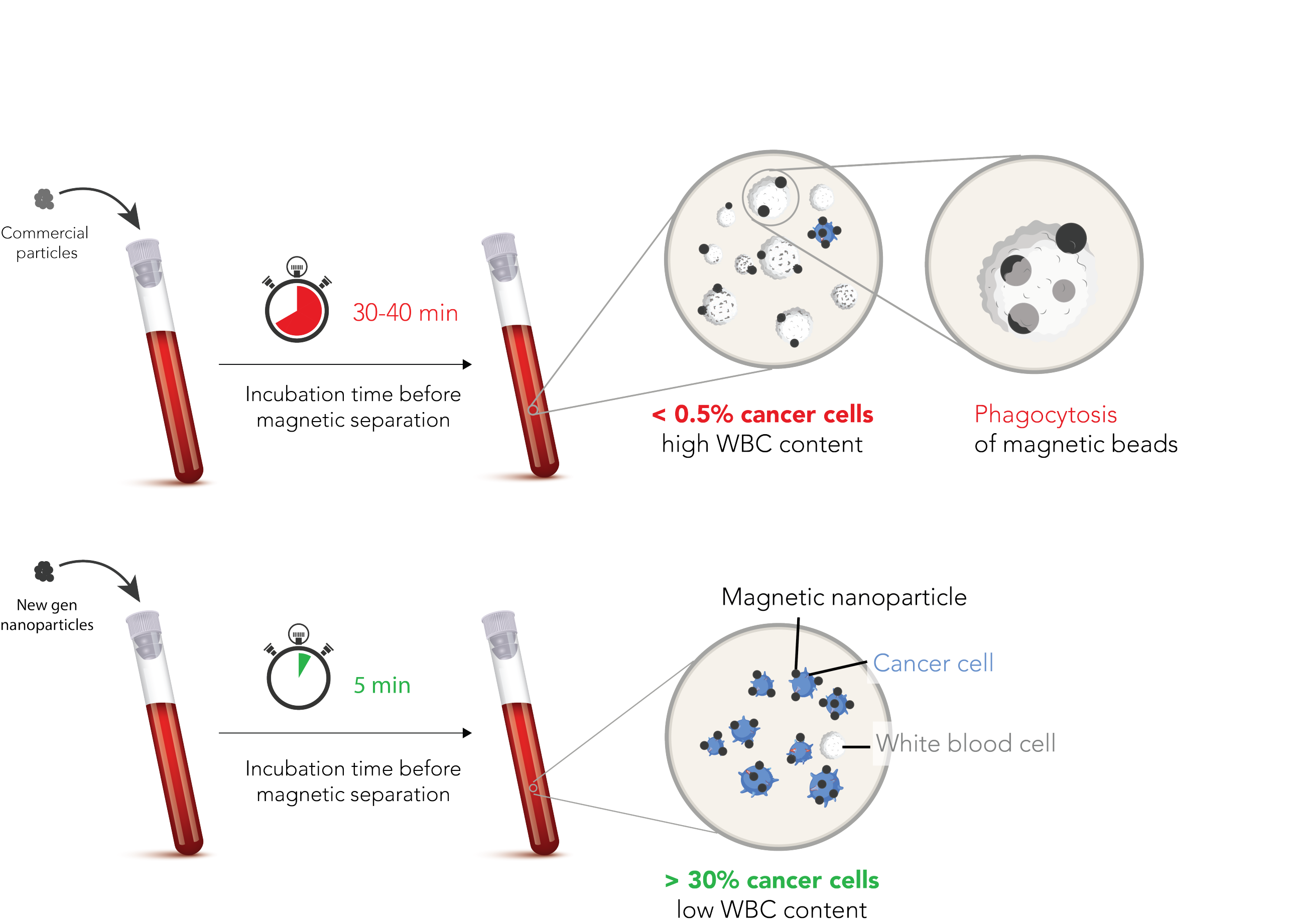
Weida: The difficulty is the rarity of CTCs in the blood. To stay with the strawberries, it’s like looking for very few strawberries in a swimming pool filled with various stuff. However, with technologies such as magnetic nanoparticles, micro- or nanofluidics, and bioinformatics, we manage to filter out the few CTCs that are in the blood and analyse them. We make use of three patented findings that came out of ETHZ and EPFL. We are the first to combine the extraction of CTCs from the blood and the preparation of samples for analysis by sequencing into a single instrument. It is necessary to combine those two steps in order to introduce CTCs to the clinic.
What keeps you enthusiastic?
Antoine: Our nanoparticles and processes are really improved. In commercial processes available so far, the nanoparticles “get eaten” by the white blood cells. Our magnetic nanoparticles survive this attack of the munchies which makes the extraction of CTCs much more effective. With improved microfluidics, we are also able to convert close to 100% of extracted cells into sequencing samples. Their analysis creates opportunities to adapt treatment – to find medication that overcomes resistance.
Weida: In addition, our technology might help to ensure patients that they are cancer-free, which can be a big relief.
Antoine and Weida: The outlook of potentially having a positive effect on people’s lives is very motivating. Collaboration has been very important – for example, CSEM is working with us on automation. By partnering with them, our process should be automated in a few months. Moreover, we have outsourced our data analysis to a bioinformatics company, and we are collaborating with big players in diagnostics. Last but not least, EPFL Professor Renaud’s expertise in nano- and microfluidics has been invaluable to us.
What are the next steps towards the market, and towards potential benefits for patients?
Antoine: We have received some funding from Innosuisse and Venture Kick and are raising a seed round now. We are in discussion with two leading oncology companies and negotiating a pilot with a major player in liquid biopsy. There is still a lengthy, costly process ahead of us. Our technology needs to undergo clinical trials, which will take about four to five years from now.
Weida: I calculate with three to five years. So hopefully, we are a bit faster and able to diagnose patients sooner. I am a bit more optimistic than Antoine (laughs).
Contact/Links:
Do you want to get more "News for Industry" stories?
external page Follow us on LinkedIn
Are you looking for research partners at ETH Zurich?
Contact ETH Industry Relations
ETH spin-offs: facts and figures
Since 1996, 496 spin-offs have been founded at ETH Zurich. ETH transfer, the technology transfer office at ETH Zurich, supports recognized ETH spin-offs in the founding process and in their first years of operation.
With the help of the Pioneer Fellowship Programme, funded by the ETH Foundation, young researchers can develop innovative products and services based on their scientific work at ETH Zurich. A Pioneer Fellowship is awarded to young ETH entrepreneurial minds intending to develop a highly innovative product or service to be exploited commercially and/or for the benefit of society.
Offers for entrepreneurs at ETH
Press release ETH spin-offs January 2022: "Battling climate change and the pandemic"