Label-free detection of tumour cells
Single cancer cells separate from their tumour and dissipate via the bloodstream through the body. Early detection of these circulating cells can help physicians to intervene in time and reduce the risk of tumour spreading. Stavros Stavrakis presents a promising diagnostic method.
Stavros, why is it difficult to detect circulating tumour cells?
It’s the needle in a haystack scenario. The tumour cells travel in the bloodstream. Typically, these cells are found at a concentration of about 1 per millilitre of blood – that also contains 100 million red blood cells, 1 million white blood cells, and other components, which will obscure the measurement. So, to detect the tumour cells, we either must purify the sample, work with ultra-high throughput, or both.
You solve the problem with a new microfluidic device. Can you tell us something about your approach?
Microfluidic devices are often referred to as lab-on-a-chip technology. Everything is miniaturised. The liquid samples are guided through micrometre-sized capillaries, which are customised to fulfil different functions. An advantage is the small sample volume required to fill these chips.
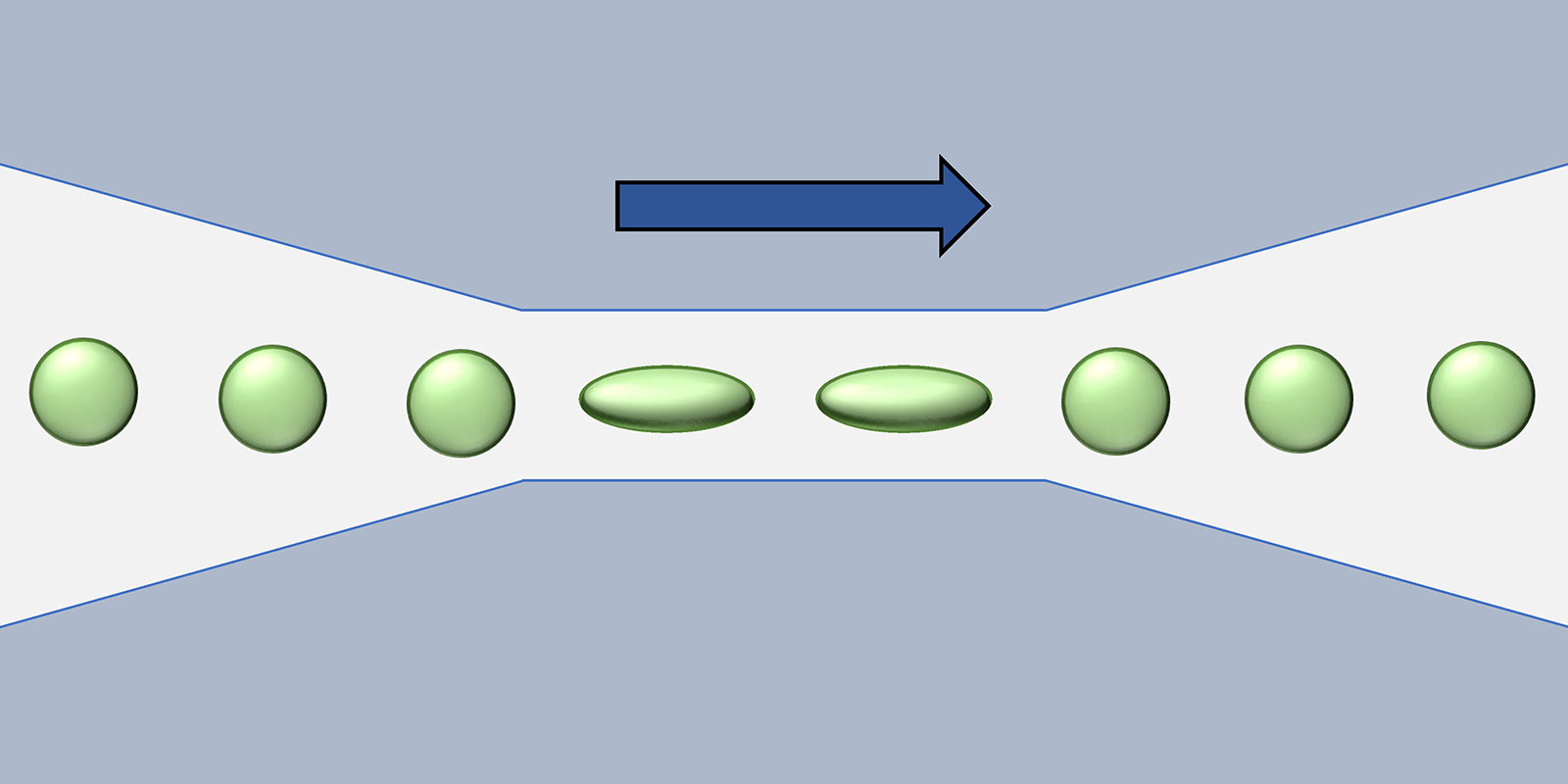
We filed a patent on a microfluidic device that measures the size and deformability of cells. Tumour cells can be identified by their deformability. Their cell membrane is less rigid than in healthy cells. This means, under pressure tumour cells show a larger change in shape.

How do you measure deformability with your microfluidic chip?
We guide the cells into a microfluidic channel that narrows down to a 15-micrometre cross-section. In the narrow section of the channel, the liquid flow speeds up and stretches the cells in the flow direction. Depending on the type of cell it shows a characteristic size-to-deformation ratio. By this ratio, we identify the tumour cells. Another benefit over other diagnostic methods is that no extra labelling of the tumour cells, such as adding fluorescence markers, is required.
And the secret of the ultra-high throughput?
Basically, we reduce friction, avoid clogging and parallelise the process. In this way, we measure 100’000 cells per second, which is two orders of magnitude faster than current state-of-the-art microfluidic deformability methods. With this ultra-high throughput, we can detect rare events such as a circulating tumour cell.
When will your technology be available to clinics?
We have already started a collaboration with the University Hospital of Zurich on a related project. Here, we apply and optimise our device to the measurement of B-cells, a subcategory of the white blood cells, for diagnosing and treating chronic lymphocytic leukaemia.

Contact/Links:
https://www.demellogroup.ethz.ch/
Further news articles from the group "Enzyme engineering for industry": https://ethz.ch/en/industry/industry/news/data/2021/06/enzyme-engineering-for-industry.html
"Applying the butterfly principle": https://ethz.ch/en/news-and-events/eth-news/news/2022/02/applying-the-butterfly-principle.html
Do you want to get more "News for Industry" stories?
external pageFollow us on LinkedIn
Are you looking for research partners at ETH Zurich?
Contact ETH Industry Relations
