Closing the gap between data science and medicine
Even after clinical trials, there is never 100% certainty about the safety and effectiveness of new medicines. The ETH spin-off Methodds offers tailored analytical solutions and expert services for this problem in healthcare.
The pharmaceutical industry is constantly developing new drugs to alleviate – and ideally cure – medical conditions. While clinical trials can identify side effects or interactions to a certain degree, testing for this is limited. However, real-world data, such as electronic health records or claims data helps better assess drug safety, potential harm, and effectiveness – and this is where Methodds excels. We spoke with the co-founders of Methodds, Stefan Weiler and Adrian Martinez de la Torre to learn more about their technology and what they are planning for the near future.
For whom did you develop this solution?
Since it can be tailored, our solution can be used by a broad range of B2B clients. We offer our analytical solutions to pharmaceutical companies, regulators, or hospitals. Private equity firms or investment funds can also use our solutions, as they sometimes have various medical companies in their portfolio and need to assess the potential and/or risk of a given medication that is being developed.
Why did you develop this combination of data and medicine?
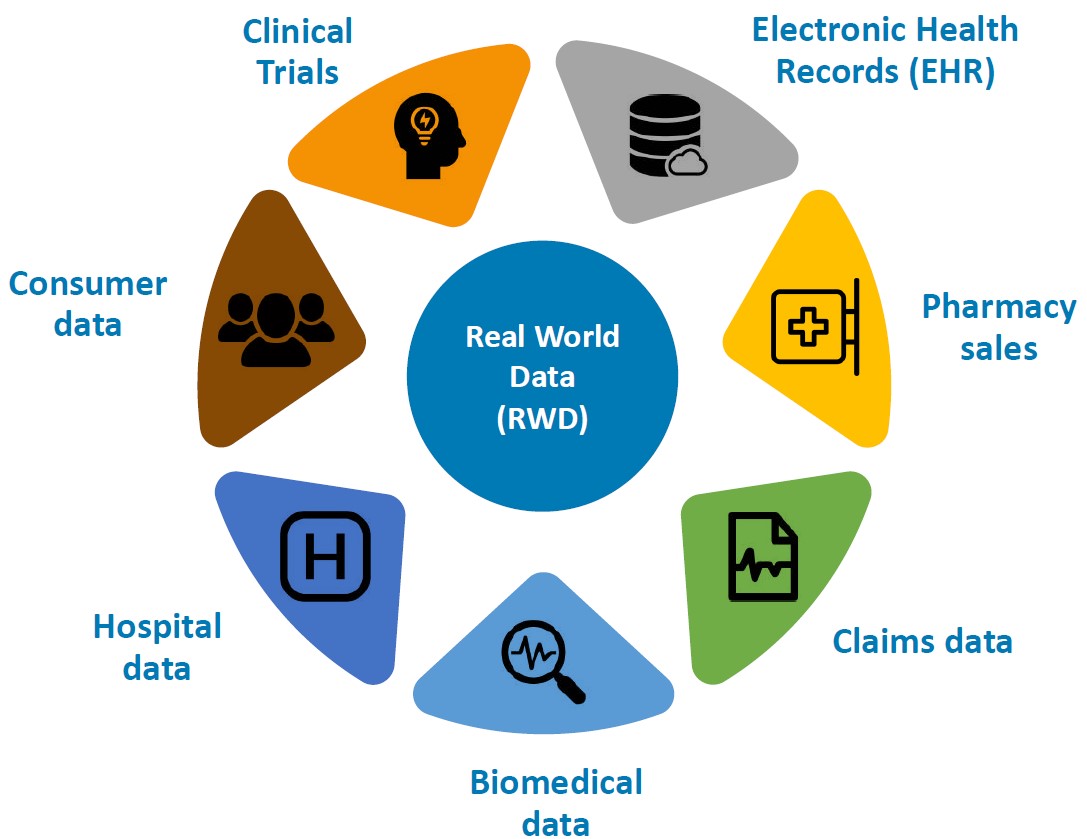
Clinical trials are performed in very controlled environments. Therefore, certain groups of people – such as pregnant women, people with disabilities, or multimorbid people with several simultaneous conditions – are usually excluded from trials. These trials are also often performed in Western countries only or are heavily biased (whether intentionally or not) towards males. Nowadays, with the advent of large electronic health records and the development of machine learning models, we can study how drugs are used at the population level. In practical terms it means that we can study the safety and effectiveness of a drug in a much broader segment of the population that were not included in clinical trials. We can create novel methods to enable the detection of harmful combinations that have never been identified or we can assess the effectiveness of marketed medications. Sometimes, we even see when drugs have positive side effects that have gone undetected before.
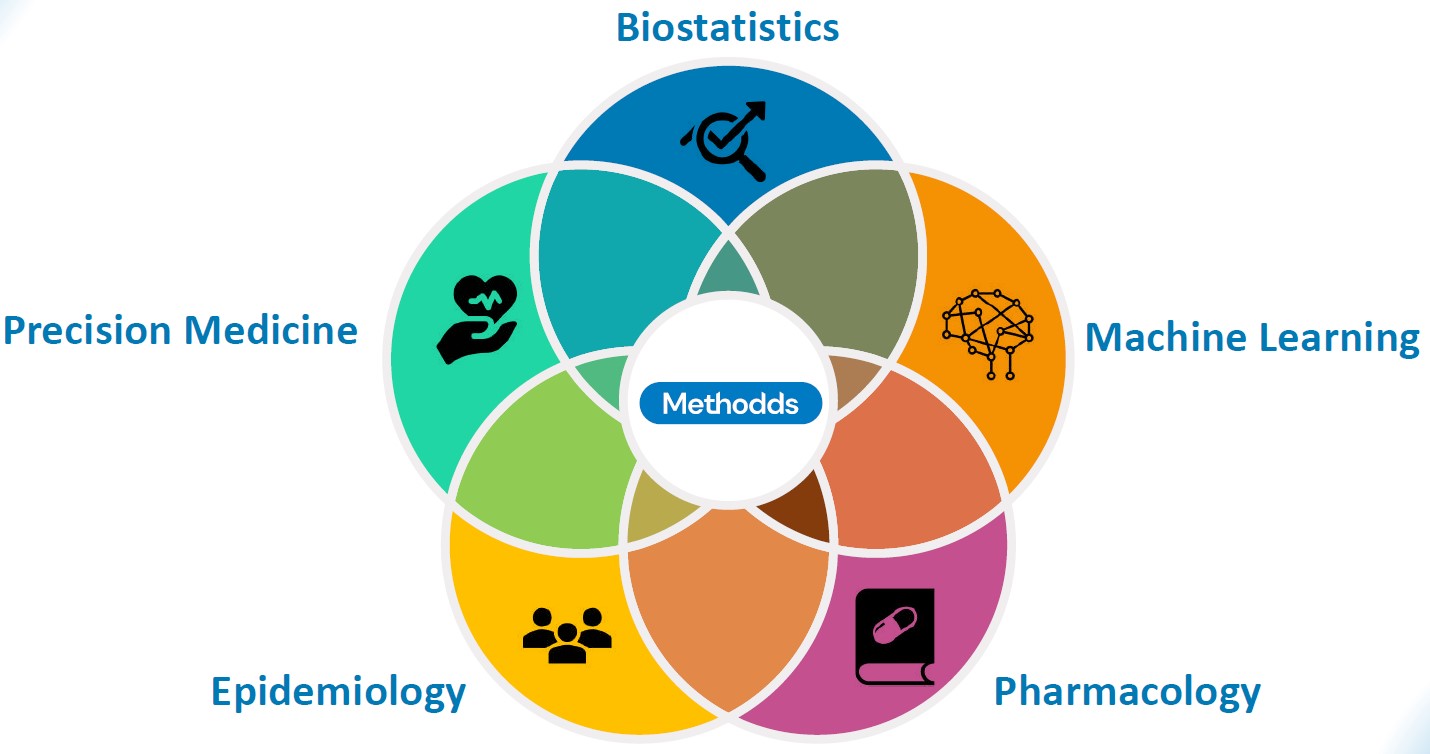
“We can assess the potential risks and benefits of medications and medical devices by using a holistic approach combined with innovative techniques.”Stefan Weiler, Co-founder of Methodds
How does your technology combine data science and medicine?
We help a given company’s medicinal product to reach its full potential. Depending on the case, we use different databases or combinations of data sources from different countries and entities such as hospitals and governments. These databases are very complex in nature and include a vast quantity of diverse information such as medical diagnoses, laboratory results, or medical prescriptions. At Methodds, using our technology and medical expertise, we combine everything into a coherent whole. We use traditional statistical methods, state-of-the-art machine learning and AI models together with strong medical expertise, thereby combining all the tools and knowledge available to offer the best solution in an interpretable way. Today, we have powerful enough computers and advanced AI models for this. Having collected all the data, we can then go back and test the evidence in medicine and check plausibility – effectively closing the gap between data science and medicine by providing tailored analytical solutions and expert services in healthcare.
“We believe these new methods of combining real-world data and machine learning with medical expertise will shift the paradigm of clinical practice.”Adrian Martinez de la Torre, Co-founder of Methodds
When will you be ready to expand and develop the product with the first clients?
We founded the company in 2022. We are looking for projects and collaborations within the pharmaceutical industry to define their needs more precisely and to offer our solutions. We are also interested in exploring projects with investment funds. In fact, we are interested in talking to all stakeholders in the healthcare realm. We believe these new methods of combining real-world data and machine learning with medical expertise to assess the potential risks or benefits of medical products will shift the paradigm of clinical practice. All stakeholders in the field of healthcare are already collecting data – with some being legally bound to do so – and this is where we can offer valuable support in data interpretation. Our goal is to improve therapeutic drug usage and reduce any potential harm thereof for the benefit of the entire society.

Contact/Links:
Do you want to get more "News for Industry" stories?
external pageFollow us on LinkedIn
Are you looking for research partners at ETH Zurich?
Contact ETH Industry Relations
ETH spin-offs: facts and figures
Since 1973, 540 spin-offs have been founded at ETH Zurich. ETH transfer, the technology transfer office at ETH Zurich, supports recognized ETH spin-offs in the founding process and in their first years of operation.
With the help of the Pioneer Fellowship Programme, funded by the ETH Foundation, young researchers can develop innovative products and services based on their scientific work at ETH Zurich. A Pioneer Fellowship is awarded to young ETH entrepreneurial minds intending to develop a highly innovative product or service to be exploited commercially and/or for the benefit of society.
Offers for entrepreneurs at ETH
Press release ETH spin-offs January 2023: Digital twins, new cancer treatments and three unicorns
