New rules of procedure to address scientific misconduct are adopted
The new rules of procedure to address scientific misconduct enter into effect on 1 June 2024 at ETH Zurich. What has changed and why a revision was necessary.
The rules of procedure in the event of suspected scientific misconduct currently in effect (RSETHZ 415) stem from the year 2004. Because these have for some time no longer satisfied today's requirements in terms of legal and technical resilience, durability and transparency, the rules of procedure were updated as part of a total revision.
Until now, the contact persons for suspected scientific misconduct were the confidants. They performed various tasks: They provided advice, answered questions, mediated conflicts relating to good scientific practice, took delivery of reports of suspected scientific misconduct and, where necessary, conducted an informal preliminary examination. As part of the preliminary examination, it was clarified whether a formal investigation by an ad hoc investigation committee was required as a second step. With this two-step process, ETH wished to prevent minor cases from immediately triggering an investigation.
But over the last twenty years, this two-step process repeatedly resulted in problems: the results of the preliminary examination were not legally binding and therefore not subject to appeal. If persons involved did not accept the result, there was no procedural path provided for them to pursue. In the event of an investigation, the investigation committee was not able to rely upon the results of the preliminary review but had to rather start all over from the beginning. Together with the time-consuming recruitment of ad hoc investigation committee members, this led to a long and very onerous process for the persons involved.
From two-step to one-step procedure
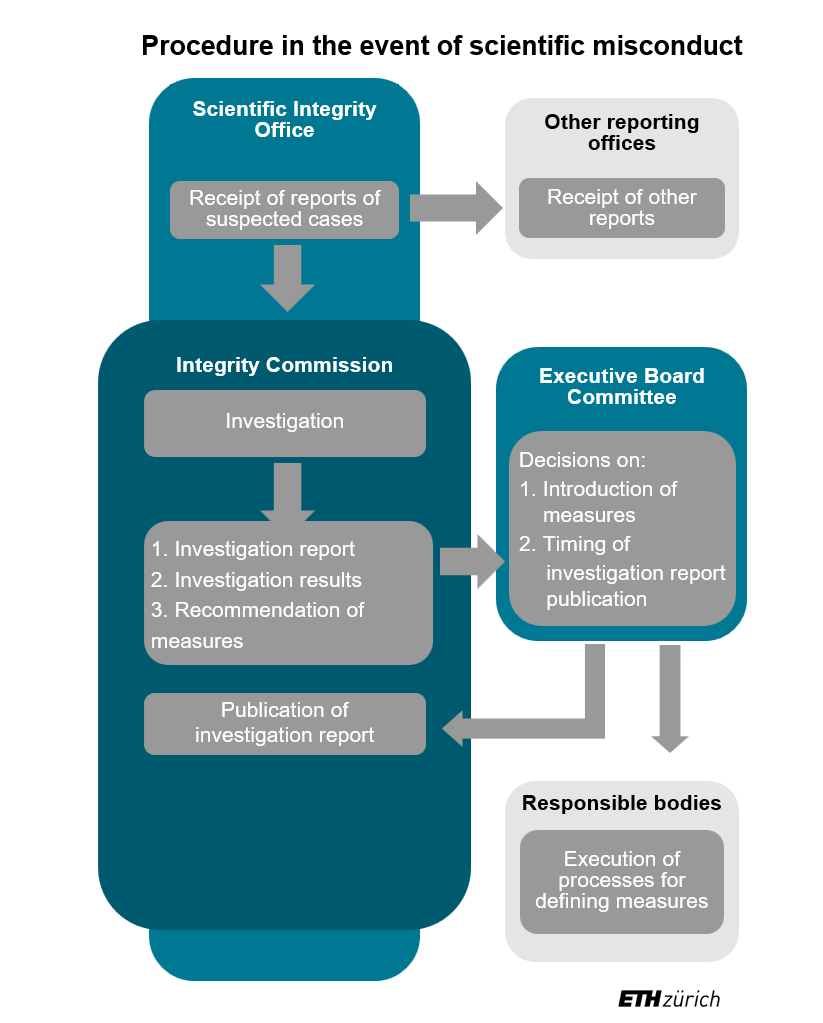
As of 1 June 2024, the procedure will be carried out through a new organisational structure. There is a standing Integrity Commission (IC) that conducts the investigations and is supported in this by a specialist unit. This newly created Scientific Integrity Office (SIO) takes delivery of the reports regarding scientific misconduct. Together with the chair of the IC, it carries out the clarification of responsibilities and triage. The definitions of scientific misconduct on which these clarifications are based were largely adopted from the external page Code of the Swiss Academies of Arts and Sciences (2021).
If, according to these definitions, there is possible scientific misconduct and corresponding evidence, the chair of the IC will initiate the investigation. A substantive investigation and assessment of whether scientific misconduct actually occurred is then only carried out by the IC, which represents a significant difference with the previous procedure.
Diverse composition of the Integrity Commission
The Integrity Commission (IC) consists of permanent members and is headed by a lawyer who has many years of experience in conducting proceedings independently. The Commission meets at least four times a year and attendance at the meetings is mandatory. If necessary, experts will be engaged to assess the content, since the members of the IC cannot cover all specialist areas. In addition, the requirements for the composition of the six to ten-member commission are high: The members elected for a period of office of four years must cover at least three of the major disciplines of Architecture & Civil Engineering, Engineering Sciences, Natural Sciences & Mathematics, System-oriented Natural Sciences and Management & Social Sciences; various genders should be represented and at least two ETH external members should be represented on the IC.
Transparency through publication of the investigation report
The Integrity Commission produces an investigation report on every investigation. The Scientific Integrity Office provides the IC with support for the investigation and preparation of the report. Following conclusion of the investigation, the IC may recommend measures to the newly established Executive Board Committee, for example on how to correct the scientific misconduct. The Executive Board Committee hears the accused again and decides on the next steps and any measures to be taken. The Vice President for Research, the Rector and the Vice President for Personnel Development and Leadership are permanent representatives on the Executive Board Committee. If the scientific misconduct involves professors, the President will also be consulted.
Once the procedure is concluded, the investigation report is published in anonymised form on the website of the Scientific Integrity Office. Publication is only dispensed with in exceptional cases that are well justified.

Raffael Iturrizaga was responsible for the revision of the rules of procedure and is head of the Scientific Integrity Office. A key task of the Office is the provision of support to the new Integrity Commission.
Dealing with possible scientific misconduct is an important issue for any university. What were the reactions to the new rules of procedure during the consultation process?
The feedback was largely positive and included constructive suggestions for adjustments, a significant number of which we were able to take into account. Most see the one-step procedure and the permanent Integrity Commission (IC) as an opportunity for a robust and significantly more effective approach, compared to today. Some were not in agreement with all definitions of scientific misconduct. But we adopted these from the Code published by the Swiss Academies of Arts and Sciences in order to provide ourselves with a uniform national basis. We were therefore unable to respond to the objections, some of which were entirely justified. But we will share the feedback with the Swiss Academies of Arts and Sciences.
Some participants in the consultation process also had the impression that the chair of the IC had too much decision-making power in relation to initiating an investigation. It is possible that there was a misunderstanding about the role of this person. At this point, they do not render any substantive assessment. They simply conduct a formal check as to whether the report relates to possible scientific misconduct and whether there is evidence that can be accessed. It is thus also important for this person to have a legal background and several years of experience in conducting administrative proceedings. Commission members may also be consulted, if necessary, e.g. for an understanding of the contents of a report.
How will advice and support in conflict situations relating to scientific integrity be ensured in the future?
Advice and prevention are key tasks for the promotion of scientific integrity. At the moment, these are carried out by different bodies. In addition to confidants, who to date have taken on the majority of operational tasks, there is also the Commission for Good Scientific Practice (GSP) that is composed of representatives from the departments. With the amendment of the rules of procedure, this interaction needs to be reorganised. The Vice President for Research has therefore commissioned the GSP Commission to develop options, with the involvement of the bodies concerned, as to how a “Research Integrity Advisory Service” (RIAS) can be established. The GSP Commission will present its proposals to the Executive Board in the spring.
The handling of conflicts, problems or disputes relating to GSP topics, for example in connection with aspects of authorship or data use, will be considered together with the ongoing revision of the regulations relating to "Reporting by members of ETH Zurich of inappropriate behaviour (RSETHZ 615)". Its revisions will likewise be dealt with by the Executive Board in the spring.
It is firmly provided for in the new rules of procedure that the investigation reports be published as a rule. Why?
One of the key elements of the total revision was to become more transparent. In addition, we also see this as an opportunity for the research community to learn from the investigations. Scientific misconduct is not always a deliberate act. Sometimes there are also situations in which researchers are unsure about the right behaviour or procedure.
Incidentally, the publication of anonymised investigation reports met with clear approval during the consultation process. There were, however, concerns as to whether suspected cases in which scientific misconduct is not confirmed should also be published. The possibility cannot be ruled out that even in the case of anonymous versions, conclusions may be drawn about the persons involved. These persons could therefore suffer unjustified harm. We have addressed these concerns in that the publication will no longer automatically take place immediately on conclusion of the procedure. On the one hand, the Executive Board Committee decides the time of publication and on the other, it may dispense with publication if there are cogent reasons. But this should remain an exception.
We will set out the contents of the proceedings whose investigation reports are not published in aggregated form in the publicly accessible annual report of the IC. The scientific community will thus not lose insight into the handling of these cases.
Who takes delivery of reports of suspected scientific misconduct until the start of the new rules of procedure?
Up until 31 May 2024, the confidants may be contacted as was previously the case. At this time, we would like to take the opportunity to thank the current and previous confidants for their work, which has been very challenging over the years and performed under conditions that were less than optimal. As of 1 June 2024, the reports will be received by the Scientific Integrity Office.
Always up to date
Would you like to always receive the most important internal information and news from ETH Zurich? Then subscribe to the "internal news" newsletter and visit Staffnet, the information portal for ETH employees.