How blood vessels grow in the brain
Thomas Wälchli’s goal is to curb the growth of brain tumours. In his doctoral thesis at ETH Zurich, the physician examined how blood vessels develop in the brain, as this process also promotes tumour growth. The scientist developed a technique that allows new insights into the specific developmental stages of blood vessels in the brain.
Blood vessels are essential for the provision of oxygen and nutrients to the body. They form a transport system through which blood passes to the individual organs and cells. In his scientific dissertation at the Department of Health Sciences and Technology (D-HEST) at ETH Zurich, the physician Thomas Wälchli examined the complex processes of blood vessel formation in the brain, which is one of the most vascularised organs in the body.
His work involved the further development of a microscopic technique that distinguishes newly forming blood vessels that are not yet perfused from perfused ones and allows their three dimensional representation. This is important because the non-perfused parts of the vessels are still growing and it is this growth process that may present new approaches for the treatment of brain tumours.
Distinct roles of blood vessels
Blood vessels serve several different functions in the brain. Their main task is to provide the brain tissue with oxygen; however, they also play crucial roles in pathological settings such as strokes, cerebral haemorrhages and tumours. Whereas new vessels contribute to the regeneration process after a stroke or a cerebral haemorrhage, blood vessels have the opposite effect in the case of tumours. “They provide the tumour cells with nutrients and oxygen, thus promoting tumour growth,” explains Wälchli, who hopes to find a way to prevent this. As a junior resident in neurosurgery at the University Hospital Zurich he frequently deals with patients with brain tumours.
For his dissertation, Wälchli studied the development of blood vessels in the brain of mice in the days following birth, when blood vessel formation is most active. With increasing age, blood vessel growth progressively slows down until the formation of new blood vessels practically ceases in the healthy brain of an adult.
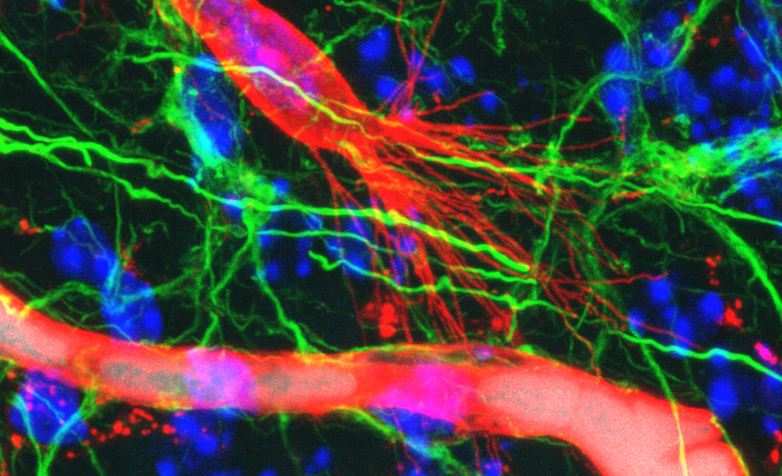
The developmental process occurs primarily in such a way that the new vessels grow from existing ones. At the front of a new vascular branch is a so-called tip cell which uses its finger-like protrusions (filopodia) to probe the surrounding tissue for signals. Depending on mechanical and metabolic feedbacks as well as on other not yet entirely understood factors, the tip cell directs the growing vascular sprout throughout the tissue and finally fuses with another vessel to form a new, perfused vascular branch.
Making blood vessels visible
The immunofluorescence method developed by Wälchli and his colleagues at the ETH, the University of Zurich and the University Hospital Zurich now allows for the first time to precisely visualise perfused and non-perfused vessels including tip cells simultaneously in a single tissue specimen. “The idea was to combine the existing imaging technique for perfused vessels with the visualisation of the tip cells,” Wälchli reports.
The method involves the following steps: first, the scientists use Evans Blue in order to make the perfused vessels visible; this dye binds to albumin, a protein found in the blood plasma. Next, a fluorescent dye that binds to the cells of blood vessel walls (endothelial cells) is used to image the non-perfused vessels. A confocal fluorescence microscope then enables detailed and contrast-rich 3D images to be made. These images are very precise and allow to identify the developmental stage of each vessel.
Using stereology, a mathematical method for 3D image analysis, Wälchli was able to calculate the volume, the length and the number of branch points of the vessels – both for the perfused and for the newly developing vessels. Furthermore, the technique also enables a precise analysis of the tip cells, including the number and length of the filopodia.
Facilitating cancer research
In the future, the method should facilitate research on signalling molecules that promote or inhibit blood vessel formation. This may, in turn, help to find treatments for diseases in which blood vessel formation plays a role. “After all, many molecules and signalling pathways that are important for blood vessel growth during brain development are reactivated during the growth of brain tumours and the regeneration following a stroke,” the physician explains.
Wälchli is currently a junior resident in the Division of Neurosurgery at the University Hospital Zurich and a Junior Group Leader at the Swiss Center for Regenerative Medicine and at the Neuroscience Center Zurich of the University of Zurich, the University Hospital Zurich and the ETH Zurich, respectively. Together with his peers, he is working on the further development of the method in order to characterise blood vessel growth in brain tumours. This would be helpful in the search for new drugs that attack blood vessels in the tumour but not in the healthy brain tissue.
Literature reference
Wälchli T, Mateos JM, Weinman O, Babic D, Regli L, Hoerstrup SP, Gerhardt H, Schwab ME, Vogel J: Quantitative assessment of angiogenesis, perfused blood vessels and endothelial tip cells in the postnatal mouse brain, Nature Protocols, 11 December 2014, doi: external page 10.1038/nprot.2015.002
Comments
No comments yet