SNF Spark for Aramesh Morteza and Xiao-Hua Qin
Spark is a new scheme for rapid funding of unconventional ideas. Launched in 2019, it has generated enormous interest. More than 700 projects were submitted by mostly young researchers with a doctoral degree.
Mini-Bone-on-a-Chip: Microfluidic Engineering of 3D Osteocyte Networks in Void-Forming Hydrogels
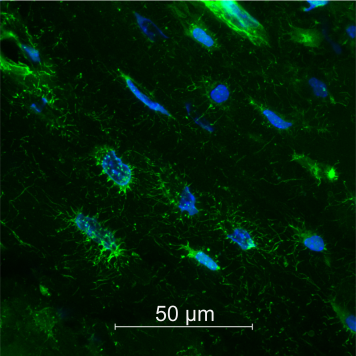
Dr. Xiao-Hua Qin, Institute for Biomechanics, Laboratory for Bone Biomechanics (Prof. Ralph Müller). His project "Mini-Bone-on-a-Chip: Microfluidic Engineering of 3D Osteocyte Networks in Void-Forming Hydrogels" is funded with CHF 100'000.
About the project:
"Osteocytes are three-dimensionally (3D) networked cells in bone that can survive for decades. They are the mechanosensors that actively orchestrate bone gain and loss throughout human life. Despite their important roles, in vitro studies of osteocyte biology remain challenging because their native environment is structurally complex, consisting of a myriad of channels and cavities running through the bone matrix as well as mechanical signals. By interfacing new void-forming hydrogels with on-chip technology, this project will establish an in vitro bone system that will enable, for the first time, the study of osteocyte network formation in 3D and long-term functionality thereof under fluid flow, which is not achievable with conventional 2D or 3D cell culture. This research will generate a new technological platform to recapitulate the physiological and pathological environments of bone tissues such as age-related bone loss."
Contact: xiaohua.qin@hest.ethz.ch
Topographical constraints for enhanced T-cell activation for immunotherapy
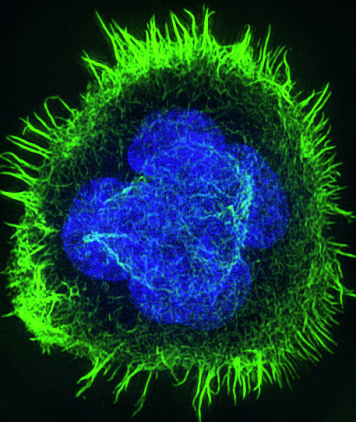
Dr. Aramesh Morteza, together with Dr. Diana Stoycheva, Institute of Translational Medicine, Laboratory of Applied Mechanobiology (Prof. Viola Vogel). Their project "Topographical constraints for enhanced T-cell activation for immunotherapy" is funded with CHF 100'000.
The project:
Adoptive immunotherapy, which is based on activation and expansion of T-cells, is an emerging biotechnology for personalized treatment of cancer. Current knowledge on the biophysics of T-cells emphasizes the importance of mechanical forces and external cues from the microenvironment as key players in the process of T-cell activation. The ability of T-cells to integrate and transduce external mechanical cues into cellular responses, is an unexplored area, which is also highly relevant to the therapeutic context. In this project we combine expertise from material sciences and biotechnology to boost activation of T-cells. It offers a new approach for the generation of potent therapeutic T-cells, which is cheap, easy, but most importantly fast. We will implement nanomaterials to control rearrangement of the cytoskeleton in T-cells, which in turn transduces mechanical cues into the cell, leading to enhanced activation. We will explore the potentials of the platform for increased number of proliferating cells compared to the existing standards.
Contact: aramesh.morteza@hest.ethz.ch