Bacteria stab amoebae with daggers
Researchers from ETH Zurich and the University of Vienna have discovered a type of bacteria that uses tiny daggers to prevent itself from being eaten by amoebae. The scientists also resolved the three-dimensional structure of the mechanism that allows the micro-daggers to be shot quickly.
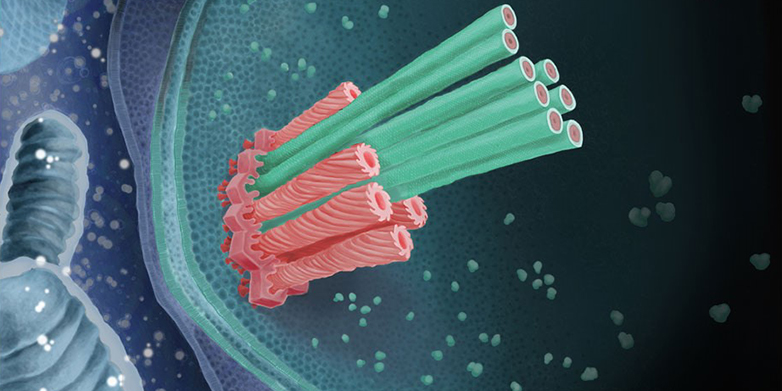
Bacteria have to watch out for amoeba. Hungry amoebae hunt them: they catch them with their pseudopodia and then absorb and digest them.
However, some bacteria know how to defend themselves. One of these is Amoebophilus, which was discovered by researchers at the University of Vienna a few years ago. This bacterium cannot only survive inside amoebae, but also thrive: the amoeba has become its favourite habitat.
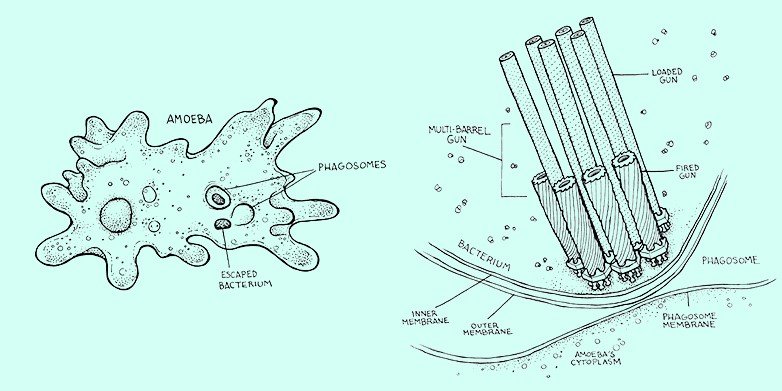
Together with the Viennese discoverers of the bacterium, scientists from ETH Zurich have now found a mechanism that they assume is crucial for the survival of Amoebophilus inside the amoeba. The bacterium has devices to shoot micro-daggers. It can use the daggers to pierce the amoeba from inside and thus escape digestion.
Escape from the amoeba’s gut
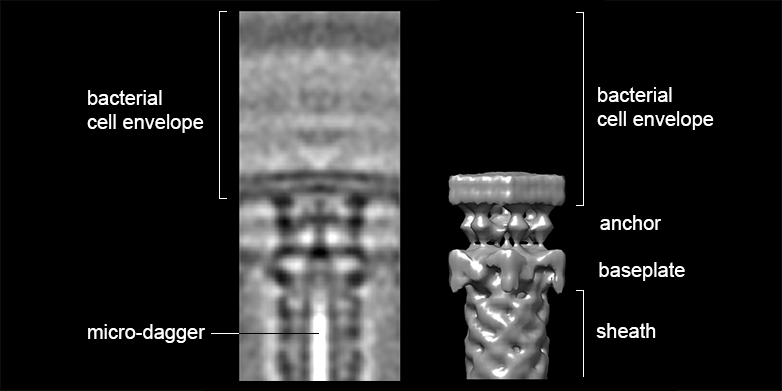
The shooting mechanism consists of a sheath attached to the bacterium’s inner membrane by a baseplate and an anchoring platform. João Medeiros, a doctoral student in Professor Martin Pilhofer’s group at ETH, explains the mechanism: “The sheath is spring-loaded and the micro-dagger lies inside it. When the sheath contracts, the dagger is shot outwards extremely quickly through the bacterial membrane.”
Bacteria absorbed by the amoeba end up in a special digestive compartment surrounded by a membrane. “Our results suggest that the bacteria are able to shoot the dagger into the membrane of the amoeba’s digestive compartment,” says Désirée Böck, also a doctoral student in Pilhofer’s group and lead author of the study published in the journal Science. This results in disintegration of the compartment, which is an inhospitable environment for the bacteria, and release of the bacteria. Once outside the digestive compartment but still inside the amoeba, the bacteria can survive and even multiply.
The process by which the digestive compartment is destroyed is not yet known. “It may be that rupture of the membrane is due solely to mechanical reasons,” says Pilhofer. However, it is conceivable that the daggers of the Amoebophilus bacteria are impregnated with a kind of arrow poison – with membrane-degrading enzymes. The blueprints for such enzymes are contained in the bacteria’s genome, as Matthias Horn, professor at the University of Vienna, and his colleagues were able to show.
Precise milling
The scientists applied a completely new method, used only by a handful of research laboratories worldwide – including that of Pilhofer – to determine the three-dimensional structure of the daggers and their shooting mechanisms at high resolution. Böck froze amoebae after they had absorbed bacteria at minus 180°C.
Much like a palaeontologist using a hammer and chisel to free fossils from stone, Medeiros then used a focused ion beam as a “nano-chisel” to work on the frozen specimens. With impressive precision, he was able to mill away the amoeba and the bulk of the bacterium, excavating the molecular daggers and their shooting devices in order to finally produce a three-dimensional electron tomogram.
First image of the overall structure
Systems related to the micro-daggers are also found elsewhere in biology: viruses that specialise in the infection of bacteria (bacteriophages) use such systems to inject their genome into microorganisms. Some bacteria can even release similar micro-devices into their surroundings to fight off competing microorganisms.
The scientists present for the first time the complete spatial structure of a shooting mechanism inside a cell in its natural context. They also show for the first time details of the baseplate and membrane anchor. “In the past, cell biologists investigated the function of such systems and structural biologists elucidated the structure of individual components,” says Pilhofer. “With the cryo-focused ion beam milling and electron cryo-tomography technologies that we have established at ETH Zurich, we can now close the gap between cell biology and structural biology.”
Multi-barrel guns
Micro-daggers had previously been found only as individual devices. In Amoebophilus, however, the scientists from Zurich and Vienna have now found apparatuses that occur in clusters of up to 30. “You could call them multi-barrel guns,” says Pilhofer.
The researchers also used genomic comparisons to investigate how Amoebophilus evolved its daggers. “The relevant genes are very similar to those of the bacteriophage injection systems,” says Pilhofer. “We assume that the genes from ancestors of today’s bacteriophages established themselves in the bacteria’s genome a long time ago.”
Also present in other bacteria
Genomic comparisons suggest that the micro-daggers occur not only in Amoebophilus, but also in numerous other bacterial species from at least nine of the most important bacterial groups. The researchers have yet to investigate whether these bacteria also use their daggers in order to avoid digestion by amoebae, or whether the daggers serve quite different purposes. They have their work cut out for a long time to come.
Finally, the scientists would like to use the new method of cryo-focused ion beam milling to elucidate the structure of other complex molecular systems. “The technique could help to address many other questions in cell, infection and structural biology. We are already working with other research groups and offering them our expertise,” says Medeiros.
Reference
Böck D, Medeiros JM, Tsao HF, Penz T, Weiss GL, Aistleitner K, Horn M, Pilhofer M: In situ architecture, function, and evolution of a contractile injection system. Science, 18 August 2017, doi: external page 10.1126/science.aan7904
Comments
No comments yet