How tumours transform blood vessels
Increasingly dense cell clusters in growing tumours convert blood vessels into fibre-filled channels. This makes immune cells less effective, as findings by researchers from ETH Zurich and the University of Strasbourg suggest.
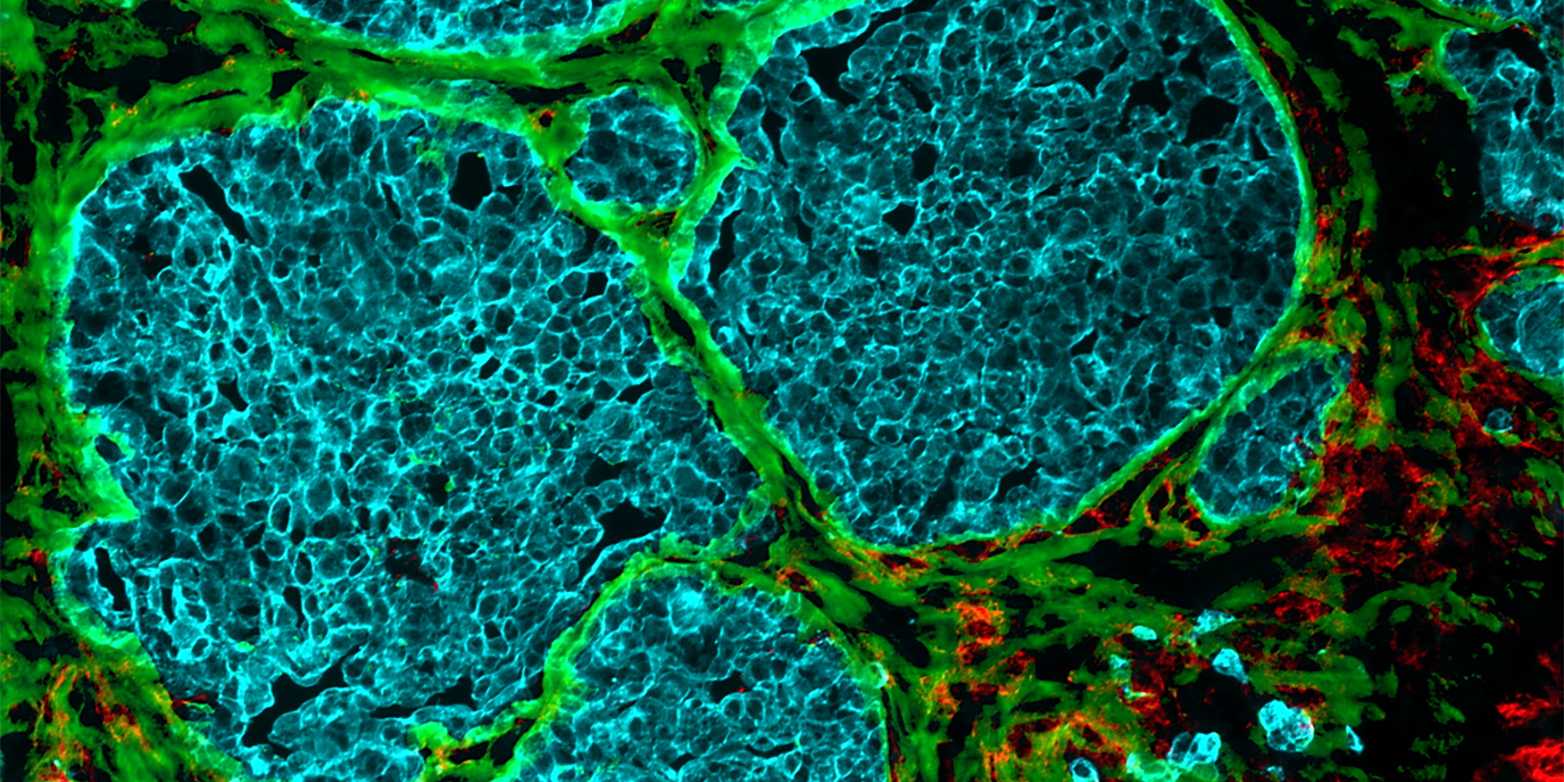
It was almost ten years ago that researchers first observed that tumours occurring in different cancers – including colorectal cancer, breast cancer and melanoma – exhibit channels leading from the surface to the inside of the cell cluster. But how these channels form, and what functions they perform, long remained a mystery.
Elaborate and detailed experiments
Through a series of elaborate and detailed experiments, the research groups led by Viola Vogel, Professor of Applied Mechanobiology at ETH Zurich, and Gertraud Orend from the University of Strasbourg have found possible answers to these questions. There is a great deal of evidence to suggest that these channels, which the researchers have dubbed tumour tracks, were once blood vessels.
These blood vessels start out by supplying the fast-growing cell clusters with glucose and oxygen. But then the vessels undergo a process that strips them of their original function of transporting blood: the vessel walls change and the vessel cavity gradually fills up.
When fibres control the behaviour of immune cells
This filler material consists mainly of cells and newly formed protein fibres, which make up what is known as the extracellular matrix. Collagen fibres are found here, as are fibronectin fibres. The latter play a role in growth processes that take place mainly during embryonic development or wound healing. In their external page article, the researchers show that the fibres within the tumour tracks are capable of trapping immune cells.
While this happens, the immune cells stretch out along the channels and stick to the loose fibronectin fibres. “In this elongated form, the immune cells switch from fighting diseases to supporting healing processes,” Vogel says. Instead of attacking the tumour cells, they excrete molecules that stimulate growth, thus helping the cancer cells to multiply.

The previously unknown role of tissue tension
It becomes clear that the tension of extracellular matrix fibers plays a key and previously unknown role in tumour development: in healthy tissue, the fibronectin fibres are stretched extremely taut; only in tumour tissue are they slack. In this looser, more relaxed form, surrounded by transformed blood vessel walls, the fibronectin fibres evidently create a recess in which cancer cells can grow undisturbed.
Vogel says that the main focus of cancer research has been on the cells: “The extracellular matrix was frequently overlooked.” That’s why the crosstalk between cells and their environment still remains a mystery. “But if you want to understand what a spider does, you also have to look at its web,” she says.
Investigating tissue samples from patients
Vogel therefore also sees the new findings as a reason to expand her research focus and gain a better understanding of the bigger picture. “The better we understand how the microenvironment steers how tumour cells multiply, the likelier it is that we’ll find a way of preventing them from doing so,” she says.
Vogel does, however, cautions to translate the implications of the results to humans because they are based on experiments on mice with breast cancer. It remains to be seen whether or not these results can be applied directly to cancers in humans. But there are indeed several parallels, as Orend’s group external page recently demonstrated.
Meanwhile, Vogel’s research group has started collaborating with the Kantonsspital Baden on a follow-up project: one of Vogel’s doctoral students is investigating if tissue samples taken from breast cancer patients also contain traces of converted blood vessels. “We’re curious to discover where we’ll find similarities and where we’ll see differences,” Vogel says.
References
Fonta CM, Loustau T, Li C, Poilil Surendranm S, Hansen U, Murdamoothoo D, Benn MC, Velazquez-Quesada I, Carapito R, Orend G, and Vogel V. Infiltrating CD8+ T cells and M2 macrophages are retained in tumor matrix tracks enriched in low tension fibronectin fibers. Matrix Biology 116: 1–27. doi: external page 10.1016/j.matbio.2023.01.002.
Murdamoothoo D, Sun Z, Yilmaz A, Deligne C, Velazquez-Quesada I, Erne W, Nascimento M, Mörgelin M, Cremel G, Paul N, Carapito R, Abou-Faycal C, Midwood K, Loustau T, and Orend G, 2021. Immobilization of infiltrating cytotoxic T lymphocytes by tenascin-C and CXCL12 enhances lung metastasis, EMBO Mol Med, 13:e13270. doi: external page 10.15252/emmm.202013270.
Comments
No comments yet