New approach for tuberculosis drugs
In the past 50 years, only one new tuberculosis drug has come on to the market, yet many more active substances are urgently needed. Current treatments increasingly fail due to multidrug-resistant pathogens. ETH researchers have now applied to patent a novel approach for developing new tuberculosis drugs.
Consumption was one of the worst known diseases of the 18th century. Thanks to medical advances, the number of deaths from this lung disease – which is today known as tuberculosis – has declined significantly. Efforts to eradicate the disease in the 1950s and 1960s resulted in a wide range of new drugs entering the market.
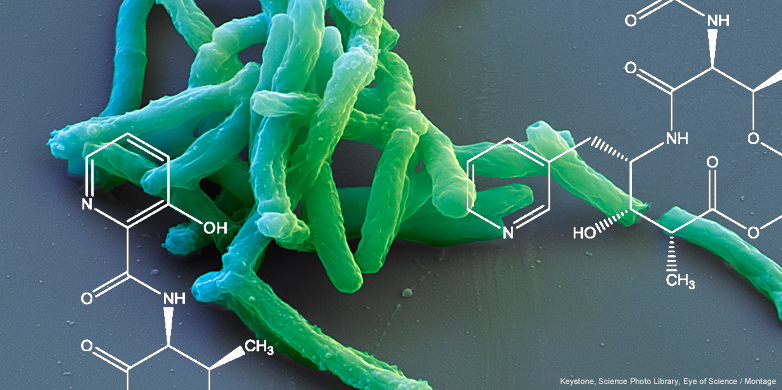
And yet 1.4 million people still continue to die each year from tuberculosis. Multidrug-resistant strains of the disease-causing pathogen are especially dangerous because they can no longer be treated with today’s drugs (see box). “In the past 50 years, only one new tuberculosis drug has come on to the market, and that was in 2012,” says Karl-Heinz Altmann, Professor of Pharmaceutical Biology at ETH Zurich. New active substances that are able to kill multidrug-resistant strains of the disease are therefore urgently needed. Altmann and his team have now laid the foundation for new tuberculosis drugs, and they were inspired by a bacteria-derived antibiotic called pyridomycin.
New design for greater efficacy
Pyridomycin inhibits the growth of the tuberculosis pathogen Mycobacterium tuberculosis, but it is degraded relatively quickly and is therefore ineffective. However, by using the structure of pyridomycin, Altmann and his research group have designed a molecule that has several advantages over the natural active substance. The new molecule is more stable and is easier to produce synthetically; it can also serve as a lead structure for the synthesis and biological testing of further modified versions of the active substance. Drugs could eventually be developed that work efficiently and are well tolerated. They could also be adapted such as to overcome new drug-resistant strains of the tuberculosis pathogen. The researchers have applied for a patent for the new active substance’s basic structure and the method of production.
The researchers found inspiration from a long forgotten substance. In 1953, Japanese scientists discovered that pyridomycin inhibits the growth of the tuberculosis pathogen Mycobacterium tuberculosis. But the compound was not investigated further for decades thereafter. “Everyone behaved as though the tuberculosis problem was solved,” says Altmann. While researching the literature, he came across the pyridomycin and worked together with a research team led by Stewart Cole, a professor of microbial pathogenesis at EPFL, to decode how it works. It paralyses an important component of cellular metabolism, which is essential for building the cell wall of the tuberculosis pathogen. Although an available drug called isoniazid targets the same key protein , it must first be converted inside the tuberculosis bacteria into the actual inhibitor. Pyridomycin, in contrast, binds directly to the target protein and therefore circumvents pre-existing resistance mechanisms that prevent isoniazid from becoming activated. The new approach of the active substance enables a wide variety of structures for new drugs against tuberculosis.
Tuberculosis resistance
Drug-resistant tuberculosis develops due to the lengthy therapy that tuberculosis patients must go through. They should take their medication for six months, but as the symptoms and discomfort usually disappear after the first few weeks patients frequently stop the therapy much too early. The result is that the pathogens remain in the body, adapt to the drugs and become resistant.
Literature references
Hartkoorn RC, Pojer F, Read JA, Gingell H, Neres J, Horlacher OP, Altmann KH: Pyridomycin bridges the NADH- and substrate-binding pockets of the enoyl reductase InhA. Nature Chemical Biology, 1 December 2013, doi: external page 10.1038/nchembio.1405
Horlacher OP, Hartkoorn RC, Cole ST, Altmann KH: Synthesis and antimycobacterial activity of 2,1'-dihydropyridomycins. ACS Med Chem Letters, 18 December 2012, doi: external page 10.1021/ml300385q
Licensing Opportunity: external page Novel synthetic scaffold for anti tuberculosis drugs
Comments
No comments yet