A new form of real gold, almost as light as air
Researchers at ETH Zurich have created a new type of foam made of real gold. It is the lightest form ever produced of the precious metal: a thousand times lighter than its conventional form and yet it is nearly impossible to tell the difference with the naked eye. There are many possible applications.
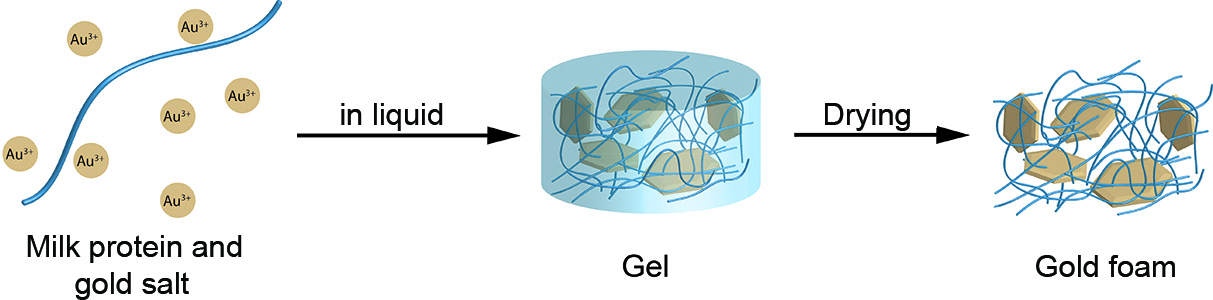
A nugget of real 20 carats gold, so light that it does not sink in a cappuccino, floating instead on the milk foam – what sounds unbelievable has actually been accomplished by researchers from ETH Zurich. Scientists led by Raffaele Mezzenga, Professor of Food and Soft Materials, have produced a new kind of foam out of gold, a three-dimensional mesh of gold that consists mostly of pores. It is the lightest gold nugget ever created. "The so-called aerogel is a thousand times lighter than conventional gold alloys. It is lighter than water and almost as light as air," says Mezzenga.
The new gold form can hardly be differentiated from conventional gold with the naked eye – the aerogel even has a metallic shine. But in contrast to its conventional form, it is soft and malleable by hand. It consists of 98 parts air and only two parts of solid material. Of this solid material, more than four-fifths are gold and less than one-fifth is milk protein fibrils. This corresponds to around 20 carat gold.
Drying process a challenge
The scientists created the porous material by first heating milk proteins to produce nanometre-fine protein fibres, so-called amyloid fibrils, which they then placed in a solution of gold salt. The protein fibres interlaced themselves into a basic structure along which the gold simultaneously crystallised into small particles. This resulted in a gel-like gold fibre network.
"One of the big challenges was how to dry this fine network without destroying it," explains Gustav Nyström, postdoc in Mezzenga's group and first author of the corresponding study in the journal Advanced Materials. As air drying could damage the fine gold structure, the scientists opted for a gentle and laborious drying process using carbon dioxide. They did so in an interdisciplinary effort assisted by researchers in the group of Marco Mazzotti, Professor of Process Engineering.
Dark-red gold

The method chosen, in which the gold particles are crystallised directly during manufacture of the aerogel protein structure (and not, for example, added to an existing scaffold) is new. The method's biggest advantage is that it makes it easy to obtain a homogeneous gold aerogel, perfectly mimicking gold alloys.
The manufacturing technique also offers scientists numerous possibilities to deliberately influence the properties of gold in a simple manner. " The optical properties of gold depend strongly on the size and shape of the gold particles," says Nyström. "Therefore we can even change the colour of the material. When we change the reaction conditions in order that the gold doesn't crystallise into microparticles but rather smaller nanoparticles, it results in a dark-red gold." By this means, the scientists can influence not only the colour, but also other optical properties such as absorption and reflection.
The new material could be used in many of the applications where gold is currently being used, says Mezzenga. The substance's properties, including its lighter weight, smaller material requirement and porous structure, have their advantages. Applications in watches and jewellery are only one possibility. Another application demonstrated by the scientists is chemical catalysis: since the highly porous material has a huge surface, chemical reactions that depend on the presence of gold can be run in a very efficient manner. The material could also be used in applications where light is absorbed or reflected. Finally, the scientists have also shown how it becomes possible to manufacture pressure sensors with it. "At normal atmospheric pressure the individual gold particles in the material do not touch, and the gold aerogel does not conduct electricity," explains Mezzenga. "But when the pressure is increased, the material gets compressed and the particles begin to touch, making the material conductive."
Reference
Nyström G, Fernández-Ronco MP, Bolisetty S, Mazzotti M, Mezzenga R: Amyloid Templated Gold Aerogels. Advanced Materials, 23 November 2015, doi: external page 10.1002/adma.201503465

Comments
Can we use it in the Jewelry gold products? How?
Excellent work. Thanks for that. What is really interesting to me, and perhaps others, is if this gold foam that you have made, may be able to resist radiation of various kinds in space beyond the Van Allen Belts? Despite many advances in the technical problem of space travel, the human factor remains the biggest impediment due to the unexpected danger of these radiations acting in concert. The radiation not only causes death and disease in large doses, but will also result in a form of severe radiation induced Alzheimer Disease, degrading the mental ability of astronauts from completing their long term mission successfully. An old article in the New York Times 1988, suggests that another kind of gold foam is special as a shield due to the exceptionally heavy nuclei. See: http://www.nytimes.com/1988/09/06/science/science-watch-gold-as-radiation-shield.html?_r=0call_made You have solar particle radiation and also galactic sources of radiation, both of these are very dangerous on long space trips to the planets such as Mars and even beyond. A light exosuit made of this form of gold woven together in plates, like "dragon skin", might be made very light while coated with a hard coating, and still be very effective due to it's piece-wise bulk. The atoms in such a very loose matrix would be capable of recoiling and absorbing the kinetic energy of high energy particles ... well mostly. What have you determined or what do you currently think about these possibilities as a radiation shield?
Dear John, thanks for pointing this out: our new gold may indeed be very suitable for this task, as the solid weight content is very high compared to the organic counter part, yet at extremely low density, which is a must in space applications! Definetely, possible outcomes of this material are way beyond our initial expectations!
If it contains 98 parts air and 2 parts solid, of which four fifths gold, then it contains about 1.6 percent gold, and it is plain impossible that it would be "a thousand times lighter than conventional gold alloys". The 1.6 percent gold would weigh more than 1/100 of conventional gold. At least one of the given numbers is incorrect. Please clarify.
The apparent density of gold in the aerogel is 0.11 g/cm3. When this is compared with the density of real gold (19.30 g/cm3) one obtains a 5/1000 times lower density. In the ETH News article, for editorial purposes, only the order of magnitude of the density ratio is given: the new form of gold is (of the order) of thousands times lower than conventional gold. For a detailed explanation please see: https://www.ethz.ch/content/dam/ethz/news/eth-news/2015/11/151125_leichtestes_gold/reply.pdf
Cool, where can I pick up my free sample :)
That last part is interesting. It is not conductive until it is squeezed together. Could the same process be used for another non-gold but conductive material? Probably.
Wonderful accomplishment! Congratulationa! Are there plans for making this material available to the public? I can imagine that it would a valuable material in the creation of works of art, in addition to its many other uses.
Isn't carat a measure of mass? So this being a 20 carat nugget it should weigh ~4g yet you claim it's lighter than air? This is extremely confusing.
Carat is a measure (in 24 units) of gold purity by mass: a 18-carat gold is a gold alloy whose mass is 75% (by weight) gold. A 24 carat gold is 100% gold. Our gold aerogel solid mass is made of 83% gold, 17% protein, and the rest is air (no weight). The total carats is then 0.83 x 24 carats = 20 carats. :-)
Can this method be used for other metals as well?
Fascinating!! Congratulations!! What about electrodes for batteries as an application?
Fair enough question. A variety of structures have been used to increase the surface area to volume ratio for electrodes, from rods to wires to foils ... so this approach has some merit, potentially. However, gold as such doesn't have any really interesting battery-like chemistry associated that I'm aware of, so I doubt this discovery would be particularly relevant for that field. But for other materials - e.g. nickel - there is certainly potential for improving the power-weight and/or capacity-volume by using sophisticated structures. Extremely fine wires have high resistance, which is not good inside a battery (generally), so you'll need several different materials in cascade.
Battery electrodes would be feasible, use the gold as a non reactive host for the anode material.